Staphylococcus epidermidis Bacteria Reduction
- Authors
-
Jessica Dobbin, BSc BA
- Facility
-
Novaerus Research and Development Labs; Ireland
- Download
- Full Report
Objective
To evaluate the efficacy of the Defend 1050 / Pro XL on reducing airborne Staphylococcus epidermidis bacteria, a surrogate for methicillin-resistant Staphylococcus aureus (MRSA).
Methodology
The test environment was a 30 m3 test chamber, located in the Novaerus microbiology laboratory. During the testing, the Defend 1050 / Pro XL device was placed inside the chamber at the centre, with the air inlet facing towards the door of the chamber. The Defend 1050 / Pro XL device was tested at maximum airflow, speed setting 5. The test chamber was controlled for temperature and humidity at 77ºF and 50% relative humidity.
Results
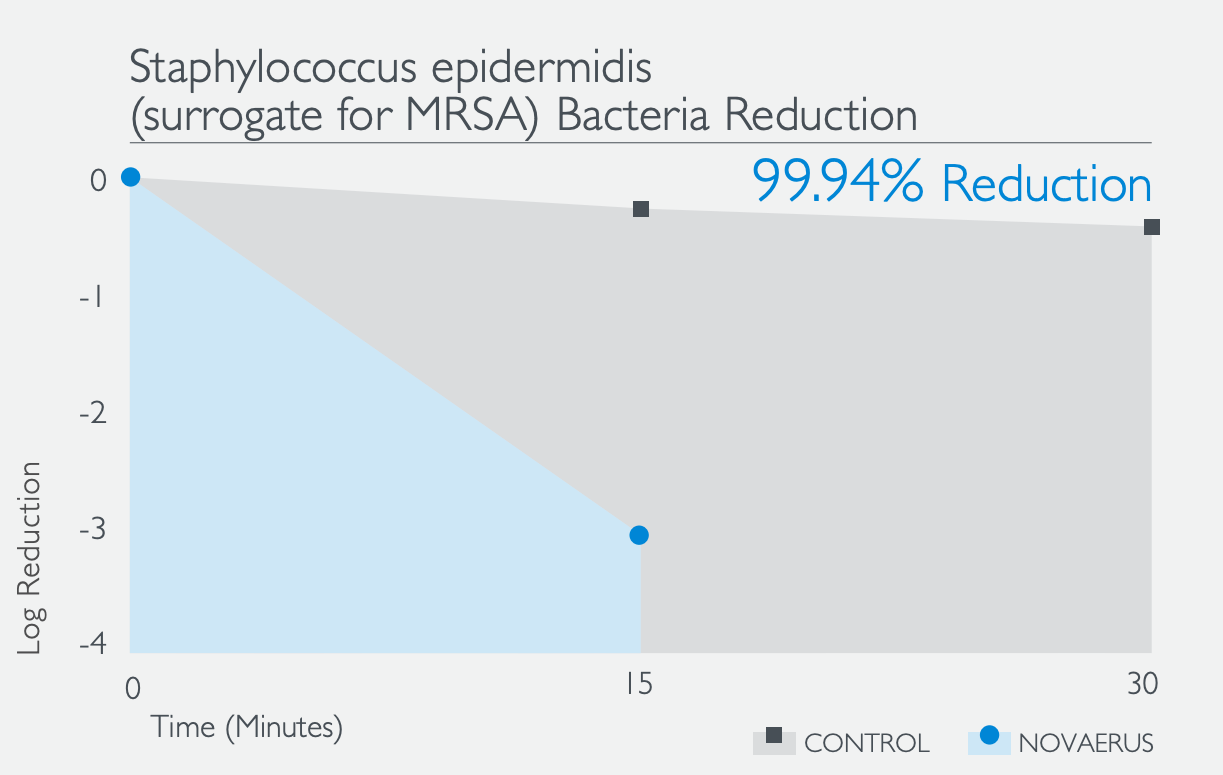
The Defend 1050 / Pro XL achieved a microbial cell reduction of 99.94% of Staphylococcus epidermidis, a surrogate for methicillin-resistant Staphylococcus aureus (MRSA), within 15 minutes of operation.
CONTACT US
USA Headquarters
Stamford, Connecticut
Call: 1 866 508 1118 Email: info@wellairsolutions.com
Ireland Headquarters
DCU Innovation Campus Old Finglas Road Glasnevin, Dublin 11
Call: +353 1 907 2750 Email: info@wellairsolutions.com