SARS-CoV-2 Reduction
- Authors
-
Jamie Balarashta - Study Director, Jeffery Trolingera - Principal Investigator
- Facility
-
Aerosol Research and Engineering Laboratories, Kansas
- Download
- Full Report
Objective
To evaluate the efficacy of the Defend 1050 / Pro XL at reducing aerosolized MS2 bacteriophage, a surrogate for SARS-CoV-2, the disease causing COVID-19.
Methodology
MS2 bacteriophage was aerosolized into a 16m3, sealed environmental bioaerosol chamber containing the Defend 1050 / Pro XL.
AGI impingers were used to sample the chamber bioaerosol concentrations. All impinger samples were serially diluted, plated and enumerated in triplicate to yield viable bioaerosol concentration at each sampling point and time. Samples were taken at 0, 15 and 30 minutes in order to quantify the reduction speed and capabilities of the Defend 1050.
Chamber control trial data was subtracted from the Defend 1050 / Pro XL trial data to yield net LOG reduction in the chamber for the bioaerosol challenges.
Summary of Results
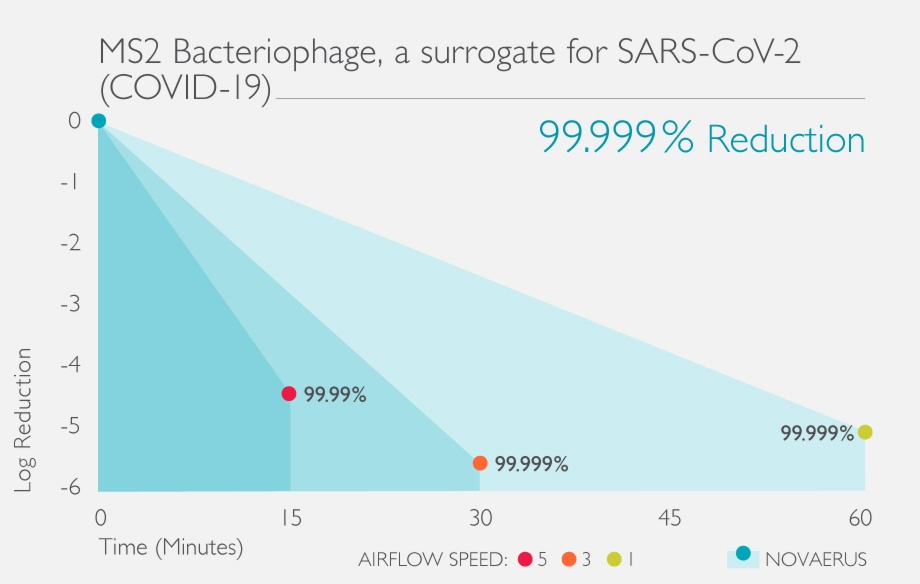
The Defend 1050 / Pro XL was shown to reduce MS2 bacteriophage by 99.99% in 15 minutes.
The Defend 1050 / Pro XL showed a high net log reduction in a relatively short amount of time with average log reduction values ranging from 4.14 net Log in 15 minutes to 4.59 net log reduction in 30 minutes.
A net log reduction over 4.0 in 15 minutes indicates the speed and efficiency of this device against the MS2 bacteriophage.
CONTACT US
USA Headquarters
Stamford, Connecticut
Call: 1 866 508 1118 Email: info@wellairsolutions.com
Ireland Headquarters
DCU Innovation Campus Old Finglas Road Glasnevin, Dublin 11
Call: +353 1 907 2750 Email: info@wellairsolutions.com