Measles Virus Reduction
- Authors
-
Tristan Russell, PhD., Senior Scientific Officer
- Facility
-
Airmid Healthgroup, Ireland
- Download
- Full Report
Objective
To assess the performance of the Defend 1050 / Pro XL in removing aerosolized Human parainfluenza type 3 (HPIV3) (renamed human respirovirus 3), a surrogate for Measles virus.
Methodology
The impact of Defend 1050 / Pro XL air purifier on aerosolized HPIV3 (strain MK-3) was conducted in a 28.5 m3 environmental testing chamber. The test chamber was preconditioned to 20 ± 3 °C and 55 ± 5% relative humidity. During testing, the chamber air handling unit was shut down, which reduces the number of air changes to as close to zero as possible.
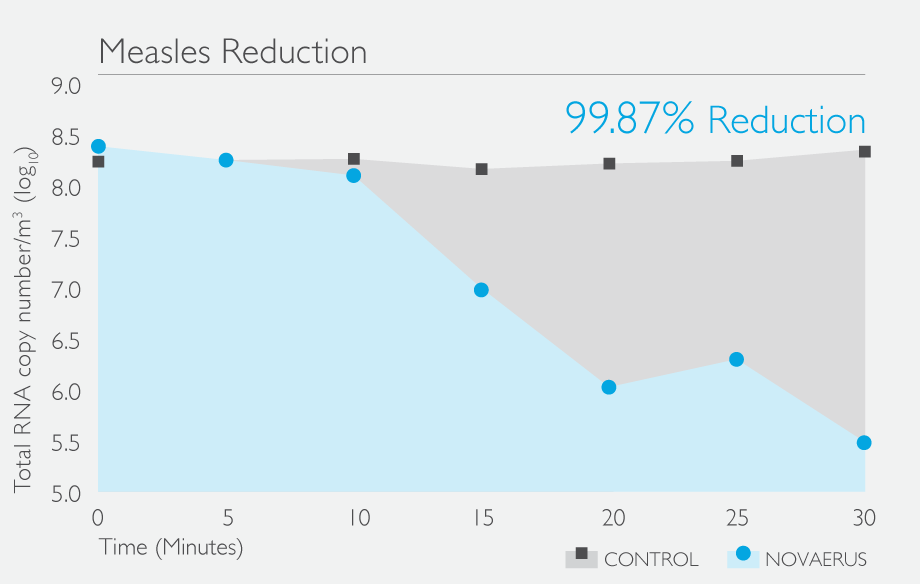
Results
The results achieved during the testing show that the Defend 1050 / Pro XL was able to reduce the concentration of HPIV3 by 99.87% in 20 - 30 minutes.
CONTACT US
USA Headquarters
Stamford, Connecticut
Call: 1 866 508 1118 Email: info@wellairsolutions.com
Ireland Headquarters
DCU Innovation Campus Old Finglas Road Glasnevin, Dublin 11
Call: +353 1 907 2750 Email: info@wellairsolutions.com